The cause of fibromyalgia still remains unknown. However, several studies are in progress aiming to find out its cause. Significant progress has been made over the last decades regarding the pathogenesis of fibromyalgia. It seems that fibromyalgia constitutes a syndrome* sharing one broad symptomatology† however not having one single cause.
*syndrome – is wider and more general term. It is set of signs and symptoms building clinical picture having various underlying causes. Disease in contrary has one specific cause usually.
†symptomatology – (1) medical science dealing with disease symptoms. (2) set of symptoms related to specific medical condition.
Hormone Rhythm Abnormality
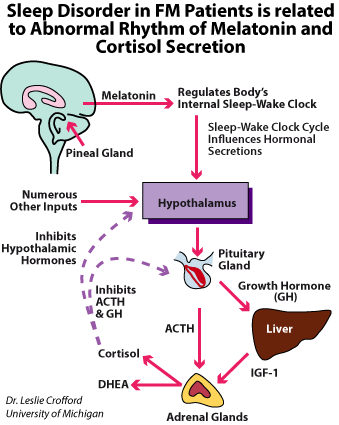
Sleep disorders are one of the most common among various symptoms of fibromyalgia. Fibromyalgia patients report early morning awakenings, awakening feeling tired or unrefreshed, insomnia, as well as mood and cognitive disturbances. Polysomnographic findings during sleep in these patients include an alpha frequency rhythm and presence of abnormal delta waves in non-REM sleep stage, so called alpha-delta sleep.
According to Bennett this could reflect in diminished production of growth hormone (GH) and insulin-like growth factor (IGF-1) that may cause improper muscle function and tiredness.
The effect of that (abnormal GH production) could be predisposition to micro-injuries in muscle fibres and inefficient repair mechanisms and subsequently weaker muscle function. The other authors suggest that the muscle weakness may result from poor energetic muscle reserve due to some amino acids like valine, leucinne, isoleucine, phenylalanine taking part in protein synthesis.Changes in circadian rhythm are observed in vast majority of patients with fibromyalgia. Pain, stiffness and mood disturbances are common symptoms in fibromyalgia. All of these conditions have the features of disrupted sleep patterns and dysregulated biologic circadian rhythms, such as stress hormone secretion. Three hormones: cortisol, melatonin and DHEA play a key role in circadian rhythm. In the physiological condition cortisol has a very distinct circadian rhythm with its lowest level late night (2 – 4 hours after falling asleep) and highest in the late morning (3 – 4 hours after waking up).
The steroid hormones, cortisol and dehydroepiandrosterone (DHEA) are the two main peripheral secretory products of the hypothalamic-pituitary-adrenal stress-neuroendocrine axis. DHEA in many respects parallels cortisol secretory activity there was some dissociation; however mean levels were positively but not tightly correlated. The secretory pattern of DHEA seems very stable.
The melatonin is a hormone produced by the pineal gland and regulates sleep and wakefulness. Melatonin in contrary is secreted at night with a robust circadian rhythm and maximum plasma levels that occur around 3 to 4 AM. The daily rise of melatonin secretion correlates with a subsequent increase in sleep propensity about 2 hours before the person’s regular bedtime.
There is a hypothesis that the abnormal rhythm of hormone levels (cortisol, melatonin, and DHEA) is responsible for the changes of circadian rhythm of fibromyalgia patients. However, the total value of the daily secretion of these hormones remains in normal range.
Sleep disturbances have been more widely presented in bookmark → sleep disorders.
Serotonin Hypothesis
In view of the evidence regarding alterations of the serotoninergic system in the pathogenesis of fibromyalgia genetic research was initially directed towards genes involved in modulation of that system. Offenbaecher et al. compared the genotype of the serotonin transporter gene (5-HTT) promoter region in fibromyalgia patients with healthy controls. An increased frequency of the S/S genotype of the 5-HTT gene was demonstrated among patients versus controls.
These results were subsequently confirmed by a study analyzing Palestinian Arabs and Israeli Jews. On the other hand, another study directed at the T102C polymorphism of the 5-HT2A-receptor gene, another serotonin receptor candidate gene, failed to demonstrate a difference in the frequency of the polymorphism among fibromyalgia patients and controls.
Similarly, a study focusing on the serotonin receptor subunit genes, HTR3A and HTR3B found no significant difference in these genes among fibromyalgia patients.
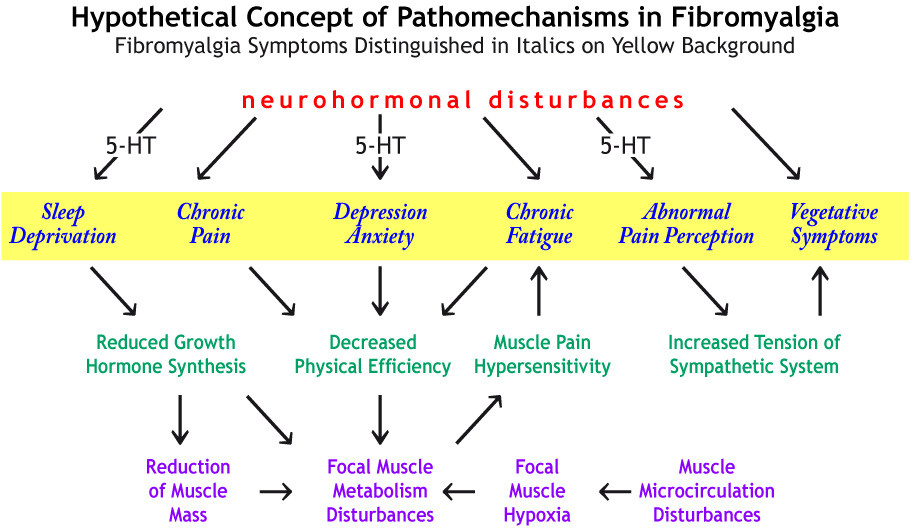
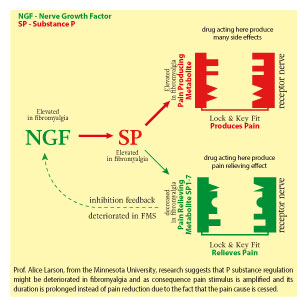
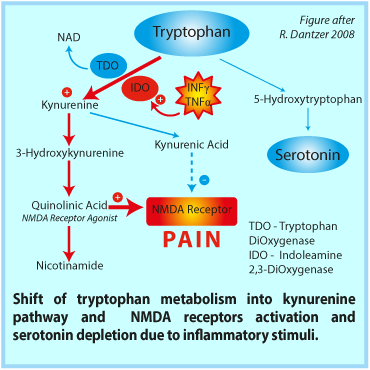
Possible links between neurochemical abnormalities and serotonin (5-HT) deficit and different FMS symptoms are presented in the figure beside. Click the link in order to magnify it → figure
Presence of 5-HT antibodies in serum and high density of serotonin receptors in nerve synapse are specified among various causes of 5-HT deficiency in fibromyalgia. Recently, a defined autoantibody pattern consisting of antibodies to serotonin (5-hydroxytryptamine, 5-HT), gangliosides and phospholipids was found in about 70% of the patients with fibromyalgia syndrome.
Kein and Berg investigated whether patients with chronic fatigue syndrome (CFS) express similar humoral immunoreactivity. Sera from 42 CFS patients were analysed by ELISA for these antibodies, and the results were compared with those previously observed in 100 FMS patients. 73% of the FMS and 62% of the CFS patients had antibodies to serotonin, and 71% or 43% to gangliosides, respectively. Antibodies to phospholipids could be detected in 54% of the FMS and 38% of the CFS patients. 49% of FMS and 17% of the CFS patients had all three antibodies in parallel, 70% and 55%, respectively had at least two of these antibody types. 21% of FMS and 29% of CFS patients were completely negative for these antibodies. Antibodies to 5-HT were closely related with FMS/CFS while antibodies to gangliosides and phospholipids could also be detected in other disorders. The observation that family members of CFS and FMS patients also had these antibodies represents an argument in favour of a genetic predisposition. These data support the concept that FMS and CFS may belong to the same clinical entity and may manifest themselves as 'psycho-neuro-endocrinological autoimmune diseases'.
Ref.:Klein R, Berg PA: High incidence of antibodies to 5-hydroxytryptamine, gangliosides and phospholipids in patients with chronic fatigue and fibromyalgia syndrome and their relatives: Evidence for a clinical entity of both disorders, Eur.J.Med.Res. 1(11):1995,21-26.